Unit 6 worksheet 5 representing ions and formula units – Embark on an enlightening journey with Unit 6 Worksheet 5: Representing Ions and Formula Units, a comprehensive guide that unravels the intricacies of these fundamental chemical concepts. This meticulously crafted resource delves into the realm of ions and formula units, empowering learners with a deep understanding of their nature, representation, and significance in chemical reactions.
Through a series of engaging and informative sections, this worksheet provides a thorough exploration of ion nomenclature, formula unit composition, ionic compound naming conventions, and the art of balancing chemical equations. Prepare to immerse yourself in a world of chemical precision and stoichiometric harmony.
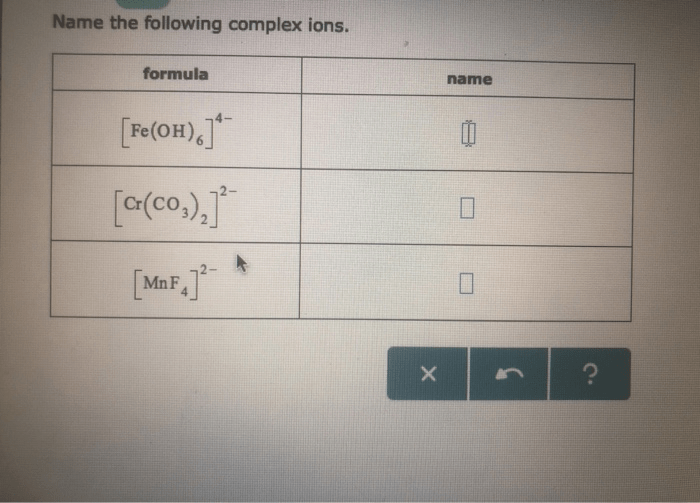
Representing Ions and Formula Units

This worksheet provides an overview of ions and formula units, including the rules for naming and representing them. It also covers writing formula units for ionic compounds, naming ionic compounds, and balancing chemical equations.
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. Formula units are the simplest whole-number ratios of ions that make up an ionic compound.
Representing Ions
Cations are positively charged ions, formed when an atom loses one or more electrons. Anions are negatively charged ions, formed when an atom gains one or more electrons.
The name of a cation typically ends in “-ium”, while the name of an anion typically ends in “-ide”. The charge of an ion is indicated by a superscript after the ion’s symbol. For example, Na+ represents a sodium cation with a +1 charge, and Cl- represents a chloride anion with a -1 charge.
Writing Formula Units, Unit 6 worksheet 5 representing ions and formula units
The formula unit of an ionic compound represents the simplest whole-number ratio of cations to anions in the compound. To write a formula unit, first determine the charges of the ions involved. Then, balance the charges by adjusting the number of ions of each type.
For example, the formula unit for sodium chloride (NaCl) is written as NaCl because the sodium ion has a +1 charge and the chloride ion has a -1 charge. The charges balance, resulting in a neutral compound.
Naming Ionic Compounds
To name an ionic compound, first name the cation, followed by the anion. For example, NaCl is named sodium chloride.
If the cation can have multiple charges, the charge is indicated by a Roman numeral in parentheses after the cation’s name. For example, Fe2+ is named iron(II) and Fe3+ is named iron(III).
Balancing Chemical Equations
A chemical equation represents a chemical reaction, showing the reactants and products involved. A balanced chemical equation has the same number of atoms of each element on both sides of the equation.
To balance a chemical equation, coefficients are added to the reactants and products. Coefficients represent the number of molecules or moles of each substance involved in the reaction.
Answers to Common Questions: Unit 6 Worksheet 5 Representing Ions And Formula Units
What is the primary objective of Unit 6 Worksheet 5?
Unit 6 Worksheet 5 aims to provide a comprehensive understanding of ions and formula units, including their representation, naming conventions, and role in chemical reactions.
Can this worksheet assist me in balancing chemical equations?
Yes, the worksheet includes a dedicated section on balancing chemical equations, providing step-by-step guidance and examples to enhance your problem-solving abilities.