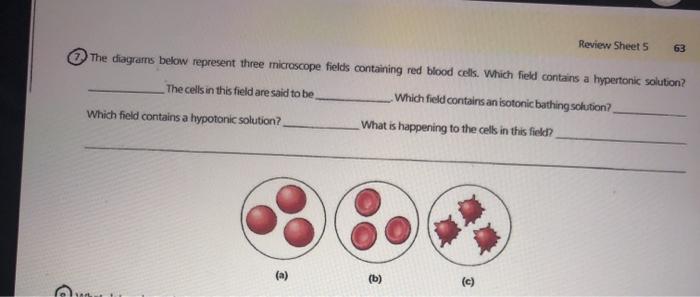
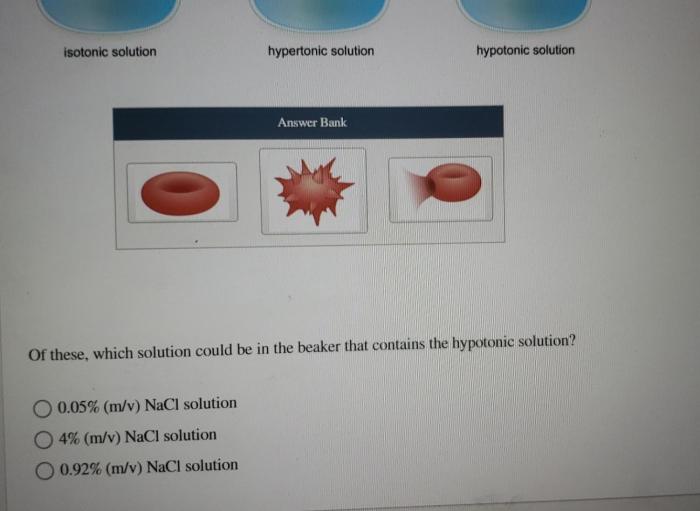
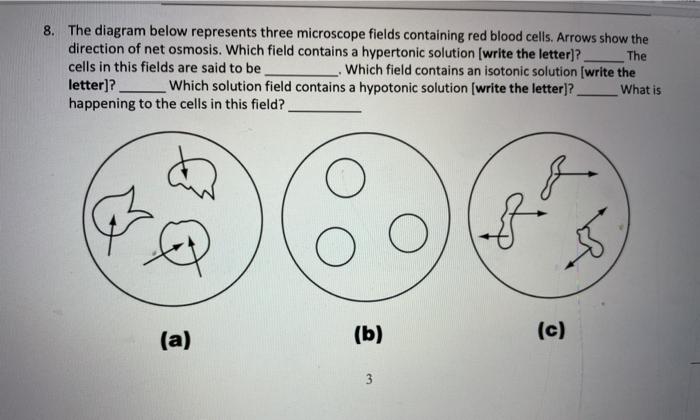
Which field contains a hypertonic solution – In the realm of science, understanding the behavior of solutions and their effects on cells is crucial. Among the various types of solutions, hypertonic solutions stand out for their unique characteristics and diverse applications. This article delves into the concept of hypertonic solutions, their identification methods, and their significance in medical and industrial fields.
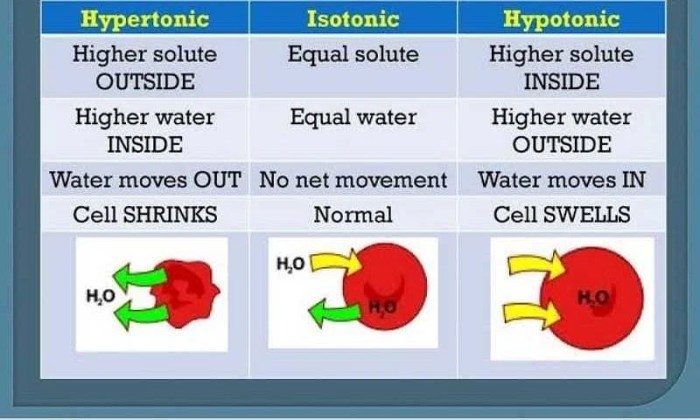
Hypertonic solutions, as the name suggests, have a higher solute concentration compared to another solution or the surrounding environment. This difference in concentration creates an osmotic gradient, leading to the movement of water molecules across a semipermeable membrane.
Hypertonic Solutions
A hypertonic solution is one that has a higher concentration of solutes (such as salts or sugars) than another solution. This difference in solute concentration creates an osmotic pressure gradient, which causes water to move from the solution with lower solute concentration (hypotonic) to the solution with higher solute concentration (hypertonic).
Hypertonic solutions can have various effects on cells. When a cell is placed in a hypertonic solution, water moves out of the cell in an attempt to equalize the solute concentration on both sides of the cell membrane. This can cause the cell to shrink and become crenated.
In extreme cases, hypertonic solutions can cause cell death.
Hypertonic solutions are found in many everyday situations. For example, seawater is a hypertonic solution relative to freshwater. This is why freshwater fish cannot survive in seawater, as their cells would lose water and shrink.
Identifying Hypertonic Solutions
There are a few different methods that can be used to determine if a solution is hypertonic. One method is to measure the osmotic pressure of the solution. A hypertonic solution will have a higher osmotic pressure than a hypotonic solution.
Another method for identifying hypertonic solutions is to measure the water potential of the solution. Water potential is a measure of the tendency of water to move from one solution to another. A hypertonic solution will have a lower water potential than a hypotonic solution.
Finally, a simple way to test if a solution is hypertonic is to place a cell in the solution and observe what happens. If the cell shrinks, then the solution is hypertonic.
Applications of Hypertonic Solutions
Hypertonic solutions have a variety of medical and industrial applications. In medicine, hypertonic solutions are used to treat dehydration and other conditions that involve fluid loss. Hypertonic solutions can also be used to draw fluid out of wounds and to reduce swelling.
In industry, hypertonic solutions are used in a variety of processes, such as food preservation and the production of chemicals. Hypertonic solutions can also be used to prevent freezing in cold weather.
Safety Considerations, Which field contains a hypertonic solution
Hypertonic solutions can be harmful if they are not used properly. Hypertonic solutions can cause dehydration, electrolyte imbalance, and other serious health problems. It is important to follow the instructions for use carefully when using hypertonic solutions.
Hypertonic solutions should be stored in a cool, dry place. They should not be allowed to freeze.
Hypertonic solutions should be disposed of properly. They should not be poured down the drain or flushed down the toilet.
FAQ Guide: Which Field Contains A Hypertonic Solution
What is the primary characteristic of a hypertonic solution?
A hypertonic solution has a higher solute concentration compared to another solution or the surrounding environment.
How do hypertonic solutions affect cells?
Hypertonic solutions cause water to move out of cells, leading to cell shrinkage and potentially disrupting cellular functions.
What are some examples of hypertonic solutions used in everyday life?
Hypertonic solutions are commonly found in sports drinks, intravenous fluids, and certain food preservatives.