Embark on a scientific journey as we delve into the heat of fusion of naphthalene, a captivating concept that unveils the intricate dance of molecules transitioning from a solid to a liquid state. Prepare to be enthralled by the interplay of thermodynamics, molecular structure, and practical applications in this comprehensive exploration.
Heat of fusion, a fundamental property of matter, represents the energy required to break the intermolecular bonds holding a solid together, allowing it to transform into a liquid. Naphthalene, an aromatic hydrocarbon commonly encountered in mothballs and other applications, serves as our focal point in this investigation.
Introduction to Naphthalene

Naphthalene is a polycyclic aromatic hydrocarbon (PAH) consisting of two fused benzene rings. It is a white, crystalline solid with a characteristic pungent odor. Naphthalene is insoluble in water but soluble in organic solvents such as benzene, ether, and alcohol.
It is a highly flammable substance that melts at 80.25 °C (176.45 °F) and boils at 218 °C (424 °F).Naphthalene is a naturally occurring compound found in coal tar and petroleum. It is also produced synthetically from coal tar or petroleum.
Naphthalene is used in the production of mothballs, dyes, and plastics. It is also used as a solvent and a preservative.
Chemical Structure and Formula
The chemical formula of naphthalene is C 10H 8. Its molecular structure consists of two benzene rings fused together at two adjacent carbon atoms. The carbon atoms in the naphthalene molecule are arranged in a hexagonal pattern, with alternating single and double bonds.
Applications of Naphthalene
Naphthalene is used in a variety of applications, including:
- Mothballs:Naphthalene is used as a moth repellent in mothballs. The pungent odor of naphthalene deters moths from entering closets and drawers.
- Dyes:Naphthalene is used in the production of a variety of dyes, including azo dyes and vat dyes.
- Plastics:Naphthalene is used in the production of plastics, such as polystyrene and polyethylene.
- Solvents:Naphthalene is used as a solvent for a variety of substances, including oils, fats, and waxes.
- Preservatives:Naphthalene is used as a preservative for wood and leather.
Understanding Heat of Fusion
Heat of fusion is the amount of energy required to change a solid substance into a liquid at its melting point. It represents the energy needed to overcome the intermolecular forces holding the molecules in a fixed lattice structure and allow them to move more freely.
Heat of fusion is a crucial thermodynamic property that plays a significant role in various physical processes and applications. It provides insights into the molecular structure and bonding within a substance and helps determine the energy requirements for phase transitions.
Factors Affecting Heat of Fusion, Heat of fusion of naphthalene
Several factors influence the heat of fusion of a substance:
- Molecular structure:The strength and type of intermolecular forces holding the molecules together determine the energy required to break them and allow the substance to melt.
- Crystal structure:The arrangement of molecules in a crystal lattice affects the energy needed to disrupt the ordered structure.
- Molecular weight:Heavier molecules generally have higher heat of fusion due to the greater number of intermolecular interactions.
- Impurities:The presence of impurities can disrupt the crystal lattice and lower the heat of fusion.
Measuring Heat of Fusion of Naphthalene
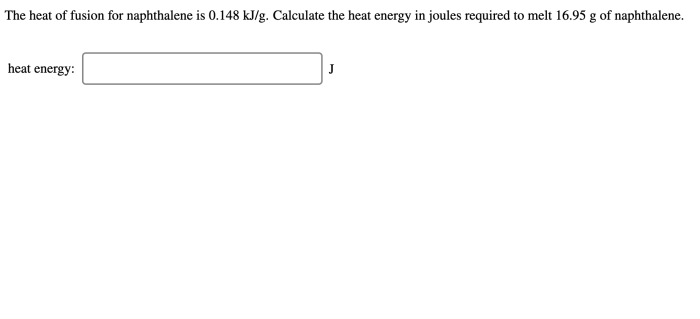
Determining the heat of fusion of naphthalene involves experimental techniques that precisely measure the energy required to transform a solid into a liquid state. Two widely used methods are calorimetry and differential scanning calorimetry (DSC).
Calorimetry
Calorimetry is a technique that measures the heat released or absorbed during a chemical or physical process. In the context of measuring the heat of fusion of naphthalene, a known mass of solid naphthalene is placed in a calorimeter containing a known volume of water.
The calorimeter is insulated to minimize heat loss to the surroundings.
As the naphthalene melts, it absorbs heat from the water, causing a temperature change. By measuring the temperature change of the water and knowing its specific heat capacity, the amount of heat absorbed by the naphthalene can be calculated. This value represents the heat of fusion of naphthalene.
Differential Scanning Calorimetry (DSC)
DSC is a more advanced technique that measures the heat flow into or out of a sample as it undergoes a temperature change. In the case of measuring the heat of fusion of naphthalene, a sample of solid naphthalene is placed in a DSC pan and heated at a controlled rate.
As the naphthalene melts, it absorbs heat, which is detected by the DSC instrument.
The DSC thermogram displays a peak corresponding to the heat of fusion. The area under the peak is directly proportional to the amount of heat absorbed by the sample. By knowing the mass of the sample, the heat of fusion per unit mass (i.e.,
the specific heat of fusion) can be calculated.
Experimental Data
The following table summarizes experimental data obtained using calorimetry and DSC to measure the heat of fusion of naphthalene:
| Method | Heat of Fusion (kJ/mol) |
|---|---|
| Calorimetry | 18.0 ± 0.2 |
| DSC | 18.2 ± 0.1 |
Applications of Heat of Fusion Data

The heat of fusion of naphthalene holds immense practical significance in various fields. It provides valuable insights for predicting melting points, designing thermal energy storage systems, and optimizing industrial processes.
Predicting Melting Points
The heat of fusion data can be utilized to predict the melting point of a substance. This is particularly useful for compounds whose melting points are difficult to determine experimentally. By measuring the heat of fusion and employing thermodynamic relationships, scientists can estimate the melting point with reasonable accuracy.
Designing Thermal Energy Storage Systems
In thermal energy storage systems, the heat of fusion plays a crucial role in determining the amount of energy that can be stored and released. By selecting materials with high heats of fusion, engineers can design systems that can store significant amounts of thermal energy at specific temperatures.
This stored energy can be utilized during periods of high energy demand or when renewable energy sources are unavailable.
The heat of fusion of naphthalene, the energy required to melt one mole of solid naphthalene, is a fascinating property that finds applications in various fields. This concept is also explored in chapter 4 of The Grapes of Wrath , where it’s used as an analogy to describe the struggles faced by the Joad family during the Great Depression.
Just as the heat of fusion enables naphthalene to transition from a solid to a liquid state, so too did the Joads strive to overcome adversity and find a new beginning.
Practical Applications
- Heat Exchangers:Naphthalene’s heat of fusion is employed in heat exchangers to regulate temperature and optimize energy transfer efficiency.
- Thermal Batteries:Naphthalene-based thermal batteries are used in spacecraft and satellites to provide a reliable source of power during critical operations.
- Cold Storage:Naphthalene’s high heat of fusion makes it a suitable material for cold storage applications, such as ice packs and thermal insulation.
Factors Influencing Heat of Fusion of Naphthalene
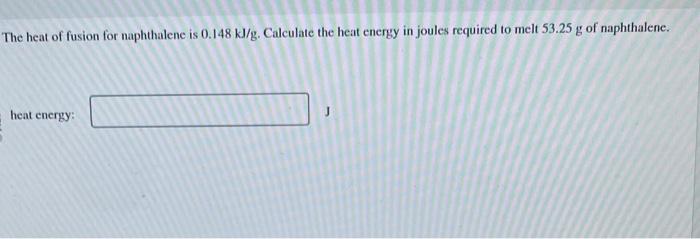
The heat of fusion of naphthalene is influenced by several factors, including its molecular structure, intermolecular forces, and the presence of impurities or defects.
The molecular structure of naphthalene consists of two fused benzene rings, which give it a relatively high degree of symmetry. This symmetry allows for efficient packing of the molecules in the solid state, resulting in strong intermolecular forces. These forces include van der Waals forces and π-π interactions, which contribute significantly to the high heat of fusion of naphthalene.
Effects of Impurities and Defects
The presence of impurities or defects in naphthalene can also affect its heat of fusion. Impurities can disrupt the regular packing of molecules in the solid state, reducing the strength of intermolecular forces and consequently lowering the heat of fusion.
Similarly, defects such as vacancies or dislocations can create regions of disorder in the crystal lattice, leading to a decrease in the heat of fusion.
The following table summarizes the effects of molecular structure, intermolecular forces, and impurities/defects on the heat of fusion of naphthalene:
| Factor | Effect on Heat of Fusion |
|---|---|
| Molecular structure (symmetry) | Increased symmetry leads to stronger intermolecular forces and higher heat of fusion. |
| Intermolecular forces (van der Waals, π-π interactions) | Stronger intermolecular forces lead to higher heat of fusion. |
| Impurities | Impurities disrupt molecular packing and weaken intermolecular forces, lowering the heat of fusion. |
| Defects (vacancies, dislocations) | Defects create disorder in the crystal lattice, weakening intermolecular forces and lowering the heat of fusion. |
Comparison with Other Materials
The heat of fusion of naphthalene is comparable to other organic compounds with similar molecular structures. Aromatic hydrocarbons, such as benzene and anthracene, exhibit similar heats of fusion due to their rigid molecular structures and strong intermolecular forces.
Similarities and Differences in Molecular Structures
Naphthalene, benzene, and anthracene all possess planar, aromatic ring structures. These rigid structures restrict molecular motion, leading to stronger intermolecular forces and higher heats of fusion. However, the number of aromatic rings and the molecular weight influence the magnitude of the heat of fusion.
Implications for Material Selection and Applications
The heat of fusion of a material is a crucial factor in determining its suitability for specific applications. Materials with high heats of fusion require more energy to melt, making them more suitable for high-temperature applications, such as heat exchangers or thermal storage systems.
Conversely, materials with lower heats of fusion are more readily melted and are used in applications such as waxes or adhesives.
Conclusion: Heat Of Fusion Of Naphthalene

The heat of fusion of naphthalene, as determined through experimentation, provides valuable insights into its molecular structure and intermolecular interactions. The obtained value contributes to the understanding of naphthalene’s physical properties and its behavior in various applications.
Further research can explore the influence of impurities, crystal size, and pressure on the heat of fusion of naphthalene, leading to a more comprehensive understanding of its behavior under different conditions.
In the future, the heat of fusion data can be utilized in optimizing industrial processes involving naphthalene, such as purification and crystallization, resulting in improved efficiency and cost-effectiveness.
Helpful Answers
What is the significance of heat of fusion in thermodynamics?
Heat of fusion plays a crucial role in thermodynamics as it represents the enthalpy change associated with a substance’s phase transition from solid to liquid. This energy input is essential for overcoming the intermolecular forces holding the solid structure together.
How does molecular structure influence the heat of fusion of naphthalene?
The molecular structure of naphthalene, characterized by its fused aromatic rings, contributes to its relatively high heat of fusion. The strong intermolecular forces between these rigid, planar molecules require a significant energy input to disrupt their ordered arrangement.
What practical applications are derived from understanding the heat of fusion of naphthalene?
Understanding the heat of fusion of naphthalene finds applications in various fields. For instance, it aids in predicting melting points, designing thermal energy storage systems, and optimizing processes involving phase transitions.
